RCSI Good Clinical Practice Day – 19th January ’24
Introduction to Clinical Research & Good Clinical Practice (from 9am) & GCP Refresher Course (from 10.20am)
Venue: Tutorial Room 4, RCSI Education & Research Centre, Beaumont Hospital
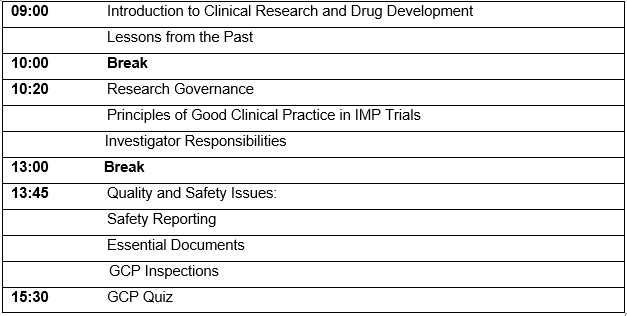
Agenda:
For further information please contact Deirdre Hyland – dhyland@rcsi.com
This ICH E6 (R2) GCP Investigator Site Training meets the Minimum Criteria for ICH GCP Investigator Site Personnel Training identified by TransCelerate BioPharma as necessary to enable mutual recognition of GCP training among trial sponsors.
Data Privacy: In order to record attendance, and to provide certification of attendance for future reference, your contact details will be retained on a course attendance record for a period of 2 years. This information will be located on the RCSI server, and will not be revealed to unauthorised persons. You may receive notification of future courses or be asked to complete audit forms. If you do not wish your data to be held, or want to have it deleted or amended at any time, this will be fully respected.
8 January 2024
SEE MORE NEWS
SEE MORE NEWS

Research Assistant – Research Nurse, Clinical Research Facility, School of Medicine, Trinity College Dublin
Post Summary This position will involve working with a team of research nurses in an established Clinical Research Facility at St James’s [...]

New report: Advancing Ireland’s Medtech Clinical Research Ecosystem: Insights, Comparisons, and Actionable Recommendations
We’re excited to share the findings of our latest report, which captures insights from Medtech stakeholders on the conduct [...]