CLINICAL TRIAL NETWORKS
CLINICAL RESEARCH NETWORKS
HRB NCTO works with and supports a number of clinical trial networks. Clinical trial networks are groups of clinicians and scientists from across Ireland who have come together around a particular disease, or clinical interest. Investigators agree on research activities that are best conducted as a large group nationally rather than in isolation.
A number of clinical trials networks have been established and are listed below by research area. Some receive state funding while others are funded mainly through charitable funds. The Health Research Board have funded cancer research through Cancer Trials Ireland since 2002 and in recent years have funded the establishment of additional Clinical Trial Networks in, Critical Care, Paediatrics (in4Kids), Primary Care, Rare Disease, Infectious Disease, Diabetes and Dementia.
The clinical research network page is live and updated on an ongoing basis. If you are a lead of a clinical research network and would like it included on the HRB NCTO website, please contact info@ncto.ie.
HRB NCTO works with and supports a number of clinical research networks. Clinical research networks are groups of clinicians and scientists from across Ireland who have come together around a particular disease, or clinical interest. Investigators agree on research activities that are best conducted as a large group nationally rather than in isolation.
A number of clinical research networks have been established and are listed below by research area. Some receive state funding while others are funded mainly through charitable funds. The Health Research Board in recent years have funded the establishment of Clinical Trial Networks in, Critical Care, Paediatrics (in4Kids), Primary Care, Rare Disease, Infectious Disease, Diabetes and Dementia.
The clinical research network page is live and updated on an ongoing basis. If you are a lead of a clinical research network and would like it included on the HRB NCTO website, please contact info@ncto.ie.
RESEARCH AREAS
RESEARCH AREAS

Cancer Trials Ireland
Cancer Trials Ireland sponsor and operate Cancer Clinical trials in Ireland and Europe. Its 50+ strong staff manage a portfolio of 100+ cancer studies. In the past 20 years almost 31,000 people have taken part in nearly 800 cancer clinical trials. In 2020, a survey of public attitudes revealed one in two people in Ireland would take part in a clinical trial.
Established in 1996, Cancer Trials Ireland (formally the All-Ireland Cooperative Oncology Research Group – ICORG) was established in 1996. It enables patients in Ireland to gain early access to novel cancer treatments and therapies. Cancer Trials Ireland operate cancer clinical trials across a number of disease areas – Breast, Gastrointestinal, Genitourinary, Gynaecology, Haematology/Lymphoma, Lung, etc. The organisation works closely with international collaborative groups such as ECOG, NSABP, ANZUP and has developed strong links with our pharmaceutical partners.
BCNI: Blood Cancer Network Ireland
BCNI is a national clinical research network set up to benefit blood cancer patients in Ireland. BCNI offer clinical trials to blood cancer patients, providing the opportunity to test new, potentially life-saving treatments and drugs. BCNI has also established a biobank and blood cancer registry, which will further our knowledge and expertise in the field of blood cancer research and ultimately improve patient outcomes. BCNI has been funded by the Irish Cancer Society and Science Foundation Ireland since 2015.
BCNI is a collaborative network of clinicians, scientists, and population health experts with a shared interest in blood cancer research. Members of the national network are based at the NUI Galway/ University Hospital Galway, University College Cork/ Cork University Hospital, Trinity College Dublin/St James Hospital, Beaumont Hospital and the Mater Hospital, and the National Cancer Registry Ireland. The key investigators involved are Dr Eva Szegezdi, Dr Philip Murphy, Prof. Mary Cahill, Prof. Michael O’Dwyer, Prof. Paul Browne, Prof. Peter O’Gorman, Prof. Kerri Clough-Gorr and Dr John Quinn
The aim of BCNI is to provide blood cancer patients in Ireland with access to novel and innovative cancer treatments through the provision of early phase clinical trials. BCNI also collect information and samples from blood cancer patients in Ireland in order to improve our understanding and to uncover new ways to combat this disease.
HRB Stroke Clinical Trial Network Ireland
Stroke is the second leading cause of death in the world, the leading cause of new disability, and a major cause of dementia and health costs.
The HRB Stroke Clinical Trial Network Ireland is led by Professor Peter J Kelly, Mater University Hospital and University College Dublin. The Network will initially involve eight Irish hospitals, six leading universities, and all seven Hospital Groups, including colleagues from UCD, RCSI, Trinity College, UCC, NUI Galway, and University of Limerick. It will have strong links with international researchers in the UK, Europe, and North America. In addition to the HRB, other Network partners are the Irish Heart Foundation, who will fund new Stroke Research Nurses, and seven industry partners, who will fund education and training activities.
In the Network, Irish researchers in hospitals will:
- Join several new international trials of new treatments for emergency care, prevention, and recovery after stroke.
- Lead a new clinical trial aiming to prevent second strokes and heart attack after first stroke.
- Train new doctors, nurses, and therapists in how to perform safe high-quality clinical trials, and will work with patient groups and the private sector to bring new treatments to patients with stroke.
HRB Irish Critical Care Clinical Trials Network

About the ICC-CTN
The Irish Critical Care Clinical Trials Network (ICC-CTN), set up in 2015 by Professor Alistair Nichol, has become a leading critical care research network in Ireland and globally. By supporting ICUs throughout Ireland and Europe, the ICC-CTN has helped to facilitate the roll-out of global trials in ICUs across the island of Ireland, giving Irish patients access to novel treatments. With a large network of collaborators around the world, the ICC-CTN has been able to secure both national and international funding, which has helped to grow the ICC-CTN team and widen the scope of the trials and research activities they conduct and support. This work has made a significant contribution to ICU research, informing evidence-based clinical practices and guidelines on a global scale.
Strategic Objectives
After receiving a renewal of funding from the HRB CTN-2021 award scheme, the network has begun the expansion of projects and activities including leadership in national and international PPIE groups, Training, Education, Methodology & more. Their main objectives are:
Research Priority Areas
The core thematic research areas include 1) Pandemic Preparedness 2) Out of hospital Cardiac Arrest, 3) Trauma, 4) Acute Respiratory Failure, 5) Acute Renal Failure. The ICC-CTN conducts and supports clinical trials and observational, non-interventional and pilot/feasibility studies in these core areas and more, including sepsis, sedation and delirium, nutrition, and physiotherapy.

During the last seven years, the ICC-CTN have been involved in the coordination of over 19 ICU clinical studies that have delivered clinical practice changing results to more than 1600 Irish patients and more than 800,000 patients globally. The network works with 18 Irish, and more than 30 European hospitals as part of a global collaboration that spans 63 countries and multiple partnerships.

HRB Dementia Clinical Trials Network
ATLANTIC DiP (Diabetes In Pregnancy)
The ATLANTIC DiP research programme has focused on examining the outcomes of pregnancy for women with Type 1 and Type 2 Diabetes and the factors influencing these outcomes. The mission of ATLANTIC DIP is to improve the outcomes of pregnancy for women with Diabetes by promoting evidence based best practice before, during and after pregnancy.
HRB Diabetes Collaborative Clinical Trial-Network-Ireland (DCCTN)
Diabetes is one of the most common chronic diseases in Ireland and the number affected by diabetes is increasing at an alarming rate alongside the increase in obesity rates. Good diabetes control and treatment avoids diabetes complications, and quality of life is sustained. Patients with diabetes throughout the course of their disease receive treatment from multiple disciplines including pharmacotherapy, surgery, medical nutritional interventions, psychology, and physiotherapy. In addition, they are exposed to new and evolving technologies to measure glucose and deliver insulin. To further advance treatments and technologies for patients with diabetes a multi-disciplinary collaborative approach is required with representation from all the departments patients will encounter.
The HRB Diabetes Collaborative Clinical Trial Network is an all-island collaborative network, the aim is to bring together key stakeholders from different disciplines to design and work on a range of ambitious multi-centre diabetes clinical trials. These trials will have the aim of improving health and wellbeing for all patients with diabetes on the island of Ireland.
It is foremost in the minds of the Network members, that all Network activities have patient and public involvement and are complimentary to the priorities of the national HSE Diabetes clinical care programme. While it is early days for the Diabetes Collaborative CTN, the core working group is working hard to increase the collaboration between members, and access for patients to clinical trials.
You can find more information about the Diabetes Collaborative Clinical Trial Network at https://diabetestrialsctn.ie/ or emailing info@diabetestrialsctn.ie
The Irish Network for VTE Research
The Irish Network for VTE Research (INViTE) is an Irish, patient-oriented research network aimed at developing and participating in excellent National and International venous thromboembolism (VTE)-related research. We are honoured to work with the patient group Thrombosis Ireland (http://thrombosisireland.ie) at every step along the way of study concept, design and development
INViTE was launched on 12th September 2018 by the Irish Minister for Business, Enterprise and Innovation Heather Humphries in Croke Park, Dublin and is a member of the prestigious INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com).
Partners
Patient organization Thrombosis Ireland (http://thrombosisireland.ie/)
Clinician organizations:
VTE Ireland; Irish Association of Emergency Medicine
Translational research groups/Centres:
UCD Conway SPHERE Research Group (http://www.ucd.ie/conway/research/researchgroups/ucdconwaysphere/); Irish Centre for Vascular Biology (http://www.rcsi.ie/ICVB)
Hospital partners:
- University Hospital Limerick
- Rotunda Hospital Dublin
- National Maternity Hospital, Holles Street, Dublin
- St Vincent’s University Hospital Dublin
- Our Lady of Lourdes Hospital Drogheda
- St James’s Hospital Dublin;
- Beaumont Hospital Dublin
- Cork University Hospital
- Mater Misericordiae University Hospital Dublin
- University Hospital Galway
- Our Lady’s Hospital Navan
- Sligo University Hospital
- Midland Regional Hospital Tullamore
- Coombe Women and Infants Hospital, Dublin
- Belfast City Hospital (Due to join in 2021)
University partners:
University College Dublin, University of Limerick, Royal College of Surgeons in Ireland, University College Cork, Trinity College Dublin, (2021: Queens University, Belfast)
CRCs: University of Limerick CRU, RCSI, UCD, TCD CRCs
Inclusion Health Service, St James’s Hospital, Dublin
Department of Health Intelligence R&D, Irish Health Services Executive
Hospital Groups: Ireland East Hospital Group
INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com)

ID-CTNI: Infectious Diseases Clinical Trials Network Ireland
ID-CTNI is led by Professor Paddy Mallon, St Vincent’s University Hospital and University College Dublin (UCD). The ID-CTNI network is hosted at the UCD Centre for Experimental Pathogen Host Research (CEPHR) and supported by the UCD Clinical Research Centre.
The ID-CTNI network brings together a team with expertise in leading, directing and assisting Infectious Diseases clinical trials and investigations, both investigator and industry led. The multidisciplinary network comprises academic clinicians and researchers from:
- University College Dublin/St Vincent’s University Hospital & Mater Misericordiae University Hospital
- University College Cork/Cork University Hospital
- Trinity College Dublin/St James’s Hospital
- Royal College of Surgeons in Ireland/Beaumont Hospital
- University of Galway/Galway University Hospital
The objectives of the ID-CTNI include:
Implement a governance structure that improves Infectious Diseases clinical trial design, methods, and coordination in Ireland
Integration of existing investigator-initiated clinical trials and the implementation of new multicentre clinical trials.
Patient involvement will play a central role in the network and planning of clinical trials.
The expertise within the ID-CTNI enables better integration of resources and training of new researchers in Infectious Diseases, this will better place ID-CTNI research internationally.
Along with other clinical trial networks, Align and integrate with national strategic initiatives in Infectious Diseases to be better prepared for future pandemics
The unprecedented focus on Infectious Diseases arising from the COVID19 pandemic is providing opportunities for the ID-CTNI to develop international clinical trials with other clinical trials networks.
Current Multi-site Clinical Trials include:
EU-COVAT-2 BOOSTAVAC (COVID-19 Booster Vaccine Trial)
An International Multicentre, Phase 2, Randomised, Adaptive Protocol to determine need for, optimal timing of and immunogenicity of administering a 4th homologous mRNA vaccination dose against SARS-CoV-2 in the general population (18+ years) already vaccinated against SARS-CoV-2
COVIRL-002 – Tocilizumab for management of severe, non-critical COVID-19 infection.
An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive decline in respiratory function and evolving systemic inflammation on time to intubation, non-invasive ventilation and/or all-cause mortality.
SWIFT Study – Semaglutide’s Efficacy in Achieving Weight Loss for Those With HIV.
A randomised, controlled, parallel group, open-label definitive intervention trial evaluating the impact of a 28-week course of weekly, subcutaneous Semaglutide alongside dietary and exercise advice on weight loss, inflammation and immune function in obese people living with HIV on stable antiretroviral therapy compared to dietary and exercise advice alone.
MPOX-VAX study – Vaccination to prevent Mpox Infection
STRIVE Trial 1 (INSIGHT 018A)
A Multicenter, Adaptive, Randomized, Controlled Trial Platform To Evaluate Safety and Efficacy of Strategies and Treatments for Hospitalized Patients with Respiratory Infections: Strategies and Treatments for Respiratory Infections & Viral Emergencies (STRIVE) – Shionogi S-217622
STRIVE Trial 2 (INSIGHT 018B)
A Multicenter, Adaptive, Randomized, Controlled Trial Platform to Evaluate Safety and Efficacy of Strategies and Treatments for Hospitalized Patients with Respiratory Infections: Strategies and Treatments for Respiratory Infections & Viral Emergencies (STRIVE) – Immune Modulation Strategy Trial (IM Strategy).
ID-NOW – Clinical Evaluation of the ID NOWTM CT/NG Test.
For queries please contact:
Dr Polina Smovolyk, Project Manager.
Email: <polina.smolovyk@ucd.ie>
Motor Neurone Disease
Research Motor Neurone (RMN) was founded in 2007, for the purpose of promoting and facilitating research into the causes and treatments of motor neurone disease (MND), also known as ALS. RMN also strives to increase awareness of this incurable disease at both a national and international level. Ongoing research is needed to discover the cause, treatment and methods of improving quality of life for MND sufferers and their families.
Neurology Research Group, St. Vincent’s University Hospital
The Neurology Research Group in St. Vincent’s University Hospital (SVUH) was established over 15 years ago. It has expertise and experience running numerous clinical pharmaceutical trials, academic research studies – both clinical and basic science, and interventional studies with allied health professionals. Several national neurology registries and research databases are also co-ordinated from the SVUH site.
Their particular areas of excellence are neuroinflammatory disorders, in particular multiple sclerosis, movement disorders and cognitive neurology. They have a dedicated clinical facility and can run clinical trials in all other areas of neurology. Their principal Investigators are recognised as international experts in their field of research and are experienced national principle investigators for many clinical trials. In addition to four consultant neurologists, they have a dedicated research team consisting of neurology research registrars, clinical research nurse manager/ coordinator, 3 clinical research nurses and a research assistant. As well as dedicated clinical facilities they have office space for external monitors, laboratory facilities on site for sample processing and access to neuroimaging, neurophysiology, neuropsychology, neuropsychiatry and neuro-ophthalmology services to name but a few. They have on-going collaborations with a number of basic neuroscience and bioengineering groups in Ireland and internationally.
For further information please contact:
Sinead Jordan, Neurology Research Nurse Manager
Email: s.jordan@st-vincents.ie
Phone: (01) 2213592

Irish Network for Children’s Clinical Trials
In4kids is a HRB funded national paediatric clinical research network hosted by the INFANT Centre in University College Cork (UCC). The network’s vision is for better medicines, medical devices & interventions for babies, children & young people in Ireland. Children are not just small adults – they have distinct physiologic, developmental, and psychological characteristics. They deserve and must have the same access to high quality, evidence-based (from paediatric studies) health care as adults..
Through increased and enhanced collaboration amongst the paediatric research community in Ireland, In4kids will develop the national capacity for high-quality, ethical paediatric clinical research.
They welcome all of the Paediatric research community across Ireland to join them, by becoming members of the network. Benefits of this are access to the members only platform of bespoke supports and tools for conducting paediatric research, increased opportunities to collaborate on national clinical research projects and being kept informed on the recent advances in national and international paediatric research.
More information on In4kids membership is available here.
Follow them on Twitter and LinkedIn.
Subscribe to their newsletter.
Visit their website for further information.
For general enquiries email: info@in4kids.ie
A research centre focused entirely on pregnancy, birth and early childhood.
INFANT is hosted by University College Cork (UCC), Ireland, and co-located in Cork University Hospital and Cork University Maternity Hospital. INFANT was established in 2013, building on over a decade of award-winning translational research with a mission to deliver pioneering translational research to improve health outcomes for mothers and babies.
INFANT’s research is underpinned by leadership in cross-disciplinary clinical and scientific research disciplines. From pregnancy to early childhood, INFANT conducts research in three core areas:
(1) Neuroscience and Development
(2) Foetal and Maternal Health and
(3) Nutrition and Growth.
INFANT has made many world-first scientific breakthroughs including: the development of an algorithm that detects seizures in new-born babies; the discovery and validation of biomarkers to detect brain injury in new-born babies, and the development of early predictive biomarkers for preeclampsia. Read more here.
Research at INFANT is led by a multidisciplinary team of investigators comprised of clinicians, scientists, and engineers with the support of the INFANT Operations and Clinical Research Teams.
Follow them on Twitter, Instagram, Facebook and LinkedIn.
Visit their website for more information.
Perioperative Clinical Trials Network Ireland
For all queries please contact:
Prof. Donal J. Buggy, Network Manager & Professor of Anaesthesiology & Perioperative Medicine, UCD.
Email: donal.buggy@ucd.ie

HRB Primary Care Clinical Trial Network Ireland
The HRB Primary Care Clinical Trials Network Ireland (CTNI) aims to support the creation of high-quality clinical evidence which improves patient outcomes in Irish primary care. To achieve this, they provide supports to researchers to develop and conduct clinical trials in primary care that address important and common problems. They then support the successful translation and implementation of this evidence into healthcare policy and practice.
The HRB Primary Care CTNI was established in 2015 as a collaborative partnership between NUI Galway, the Royal College of Surgeons in Ireland, Queen’s University Belfast, the Association of University Departments of General Practice in Ireland, and the Irish College of General Practitioners. Since establishment, it has supported more than 30 clinical studies, recruited almost 4,000 patients, leveraged funds of over €19,000,000, and published in leading international journal such as the New England Journal of Medicine, the Lancet, and the British Medical Journal.
In 2021, the HRB Primary Care CTNI was successful in securing funding for the next 5 years of operations, with a focus on achieving the following strategic objectives:
- To maximise the successful delivery of primary care trials in Ireland.
- To continue to build capacity for world-class clinical trials in Irish primary care, through the provision of financial supports to early career researchers, the promotion of primary care research education and dissemination, and the provision of seed funding to develop a roadmap for implementation of a national research IT infrastructure.
- To develop an agenda for Irish primary care clinical trials research, by leading a priority setting partnership to develop the ‘Top 10’ research priorities in chronic disease management, and a core outcome set (COS) for future trials in this area.
- To enhance patient and public involvement (PPI) in primary care research in Ireland, by continuing to grow the capacity of the Primary Care CTNI PPI group, including PPI in network oversight and portfolio development, and continuing our synergistic relationship with the national PPI network.
For more information, contact the Primary Care CTNI directly by emailing primarycaretrials@universityofgalway.ie
Irish College of General Practitioners (ICGP)
The research unit of the ICGP aims to develop and support research and audits in general practice. The overall aim of the research unit is to contribute to the knowledge base of general practice and to support evidence-based practice. The ICGP Research Ethics Committee, granted formal recognition on 17th May 2005, reviews both CTIMP and non-CTIMP projects. The College provides research training and guidance to GPs and GP trainees through in a variety of formats and is a partner on the Primary Care Clinical Trials Network.
For all queries please email: research@icgp.ie
HRB Rare Disease Clinical Trial Network:
The RD-CTN, a HRB research network, led by Prof Rachel Crowley and Prof Cormac McCarthy at University College Dublin, aims to enhance rare disease care and outcomes, with the following objectives:
- Act as a collaborative hub for trials in rare diseases
- Facilitate and support the conduct of trials in rare diseases
- Increase the opportunities for rare disease patients to access high-quality clinical trials.
This will result in increased in patient engagement; more trial opportunities for rare disease patients; increased opportunity for investigator-initiated trials; significant industry investment into Irish healthcare; enhanced knowledge of rare disease through patient focused research and parallel translation scientific studies.
Link to HRB announcement: https://www.hrb.ie/news/news-story/article/hrb-announces-e1-million-investment-for-rare-disease-clinical-trial-network-on-rare-disease-day-2022-1/
Website: rarediseaseresearch.ie
Current Clinical Trials:
TOPAZ:
TOPAZ is a randomised open-label clinical trial for people with osteogenesis imperfecta (OI). Osteogenesis Imperfecta (OI) is a rare genetic condition present from birth and is frequently called ‘brittle bone disease’. The study aims to investigate whether a treatment with a drug called teriparatide (TPTD) followed by treatment with another drug called Zoledronic acid (ZA) reduces the risk of broken bones occurring in people with OI.
Study design:
Participants will be randomised in a 1:1 ratio to receive treatment with teriparatide (TPTD) for 2 years followed by an infusion of ZA or to receive standard care for the duration of the study. All participants will be followed up for a total duration of between 2 and 5 years.
Inclusion criteria:
- Adult patient aged 18 years and over with a clinical diagnosis of osteogenesis imperfecta.
- Patients willing and able to consent and comply with the study protocol
Type of Study: Phase III/IV
Study Status: Ongoing
Principal Investigator: Prof Rachel Crowley
Contact: Suzanne.mccormack@ucd.ie
IMPALLA-2:
IMPALLA – 2 is a randomized, double-blind, placebo-controlled multicentre clinical trial investigating efficacy and safety of inhaled molgramostim (rhGM-CSF) in patients with Autoimmune Pulmonary Alveolar Proteinosis aPAP. Autoimmune PAP is caused by an immune system malfunction due to IgG antibodies that block the granulocyte-macrophage colony stimulating factor (GM-CSF) effect. GM-CSF is a protein that regulates clearance of surfactant (a mix of protein and fat) by alveolar macrophages. The surfactant accumulates in the air sacs of the lungs and eventually lead to an inability to breath. The aim of this trial is to confirm if Molgramostim Nebulizer Solution (inhaled molgramostim) improves function of the lungs in patients with autoimmune pulmonary alveolar proteinosis (aPAP).
Study design:
The trial will include 2 periods; a double-blind treatment period consisting of up to 8 trial visits and an open-label follow-up period consisting of up to 5 trial visits.
Type of Study: Phase III/IV
Study Status: Ongoing
Principal Investigator: Prof Cormac McCarthy
Contact: Suzanne.mccormack@ucd.ie
__________________________________________________________________________________________
For any other query, contact:
Suzanne McCormack
Rare Disease Clinical Trial Network Manager
Email: Suzanne.mccormack@ucd.ie
Tel: 086 8573927
The Rare Kidney Disease (RKD) Registry and Biobank
The Rare Kidney Disease (RKD) Registry and Biobank was established in 2012 to facilitate research into the rare auto-immune condition ANCA-associated vasculitis (AAV) in Ireland. Development of a networked collaboration across all the major hospital sites in the country enabled effective recruitment of patients presenting with the disease, and the capture of their clinical data and linked blood and urine samples. After joining the pan-European initiative ERN-RITA in 2022, the resource was renamed the RITA-Ireland Vasculitis (RIV) Registry and Biobank, contributing data to the RITA registry. Hosted within the School of Medicine at TCD, the Registry and Biobank today comprises over one thousand vasculitis recruits, many of whom have been tracked longitudinally, and represents one of the largest bioresources of it’s kind globally. The Principal Investigator is Professor Mark Little.
Find out more at https://www.tcd.ie/medicine/thkc/our-research/rita-ireland-vasculitis-riv-registry-and-biobank/ & https://ern-rita.org/rita-registry/general-info/
Respiratory and Asthma Research Network
The INCA™ Studies are a suite of clinical investigations. The focus of our research is in developing a novel technology which can be used in clinical practice as an objective assessment of patient adherence to inhaled therapy.
For further information please contact:
Elaine Mac Hale, Programme manager: Elainemachale@rcsi.ie
Arthritis Research Coalition
A recent report assessing rheumatology research excellence, measured by the number of citations per article published between 1996 and 2010, ranked Ireland 1st of the 35 richest countries. Major research outputs have included molecular studies on the pathogenesis of common rheumatic diseases, the discovery and validation of prognostic biomarkers, and clinical studies on novel therapies. These have relied on the recruitment of highly characterized patients populations and by the development of linked biorepositories of blood and synovial tissue.
Despite these successes the research activity has been reliant on a fragmented and poorly funded infrastructure. The development of national rheumatology research networks in many European countries including Britain, Sweden and The Netherlands highlights their value. The establishment of Arthritis Ireland chairs, and the development of a Dublin Centre of Excellence, will form the foundation of national rheumatology research strategy, however, the development of a research nurse network will seek to engage all clinicians, co-ordinate all research activities and ensure that Ireland remains on top.
The primary aim is recruit patients with common rheumatic diseases. and obtain biosamples that will underpin clinical research. A secondary aim is to increase national involvement in clinical trials of novel therapeutic agents.
The consortium lead is Prof Gerry Wilson. The leads by area are as follows:
Rheumatoid Arthritis – Prof Doug Veale
OA/Crystal arthritis – Prof Geraldine McCarthy
Spondyloarthropathies – Dr Barry O’Shea
Connective Tissue Diseases – Dr Grainne Murphy
Paediatrics – Dr Orla Killeen
Metabolic Bone- Dr John Carey
For further information:
Email: rheumatology@ucd.ie
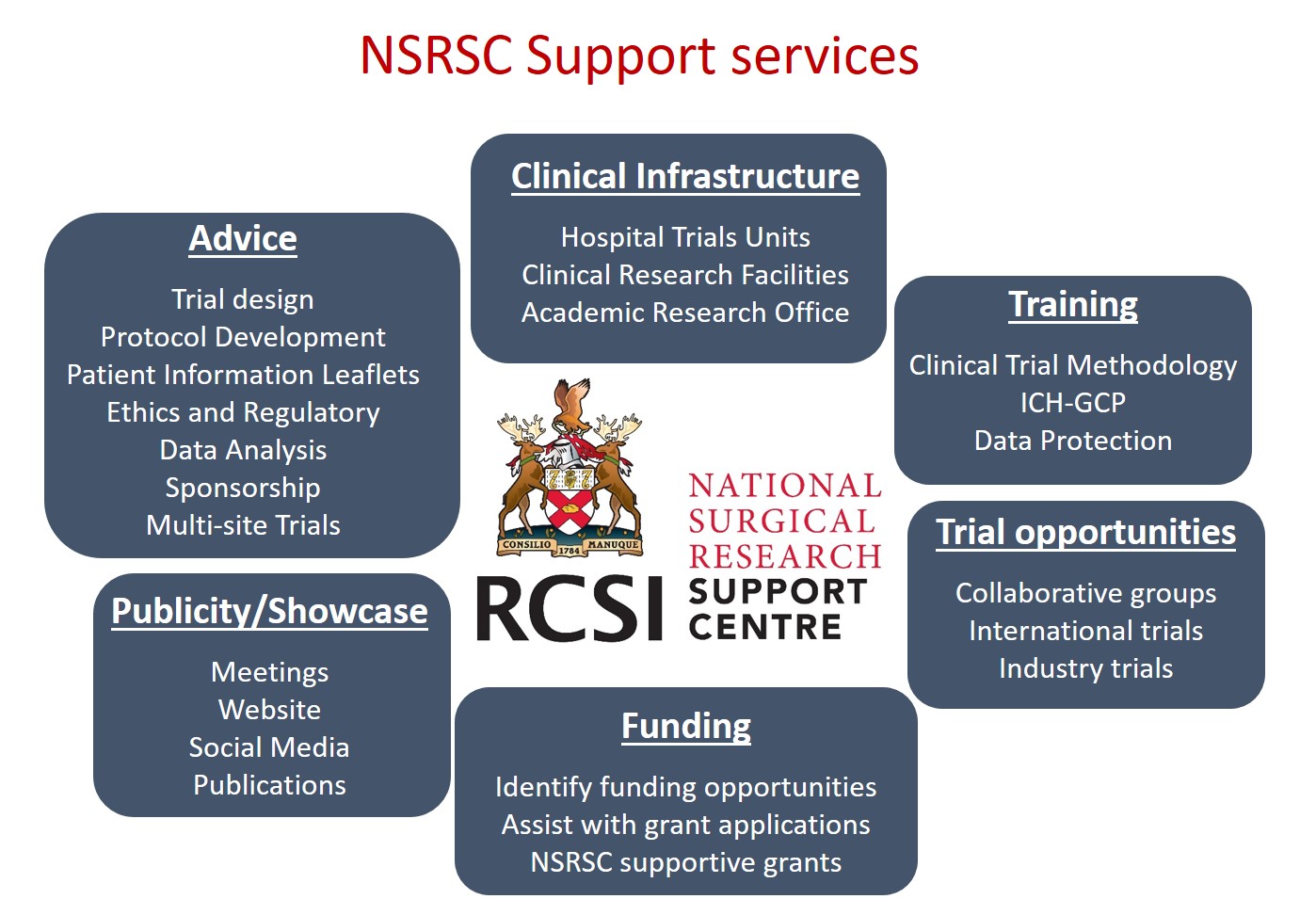
National Surgical Research Support Centre (NSRSC)
The RCSI-NSRSC was set up to establish an all-island Irish Surgical Trial Network to enhance the collaboration and coordination of surgical research and trial activity in Ireland.
Objectives of the Network
Encourage and facilitate surgical trials and research to embed a culture of research in clinical practice.
Establish a trial portfolio to generate high quality evidence to inform policy and set professional standards & guidelines for Irish surgery to improve patient outcomes.
Provide training and education to surgical trainees to produce the next generation of academic leaders in surgery.
Showcase surgical trial activity to establish Ireland as an attractive place to conduct innovative surgical trials.
To provide every patient access to high quality surgical trials and innovative cutting-edge technology across the island of Ireland.
Supports offered
The NSRSC acts as a point of contact, local sponsor and trial coordinating centre for Investigators and international sponsors who would like to open trials here in Ireland. We offer access to a wide network of surgeons, surgical trainees, allied healthcare professionals and scientists participating in surgical research.
Types of surgical research supported
The centre supports trials across all surgical specialities and thematic areas.
Currently the NSRSC are supporting multi-centre Investigator-led Randomised Controlled trials across a range of areas including diabetic foot disease, appendicitis, bariatric surgery, wound healing devices, varicose vein surgery, cancer and chronic subdural haematoma.
One key area of focus is on infection prevention in patients undergoing a variety of surgical procedures.
The network also supports and coordinates international collaborative surgical trials, pilot/feasibility studies, observational and non-interventional research studies.
Check out the website, Twitter and LinkedIn or contact the NSRSC:
Web: www.rcsi.com/NSRSC
Twitter: @RCSI_NSRSC
LinkedIn: www.linkedin.com/company/nsrsc
Email: NSRSC@rcsi.ie

Cancer Trials Ireland
Cancer Trials Ireland sponsor and operate Cancer Clinical trials in Ireland and Europe. Its 50+ strong staff manage a portfolio of 100+ cancer studies. In the past 20 years almost 31,000 people have taken part in nearly 800 cancer clinical trials. In 2020, a survey of public attitudes revealed one in two people in Ireland would take part in a clinical trial.
Established in 1996, Cancer Trials Ireland (formally the All-Ireland Cooperative Oncology Research Group – ICORG) was established in 1996. It enables patients in Ireland to gain early access to novel cancer treatments and therapies. Cancer Trials Ireland operate cancer clinical trials across a number of disease areas – Breast, Gastrointestinal, Genitourinary, Gynaecology, Haematology/Lymphoma, Lung, etc. The organisation works closely with international collaborative groups such as ECOG, NSABP, ANZUP and has developed strong links with our pharmaceutical partners.
BCNI: Blood Cancer Network Ireland
BCNI is a national clinical research network set up to benefit blood cancer patients in Ireland. BCNI offer clinical trials to blood cancer patients, providing the opportunity to test new, potentially life-saving treatments and drugs. BCNI has also established a biobank and blood cancer registry, which will further our knowledge and expertise in the field of blood cancer research and ultimately improve patient outcomes. BCNI has been funded by the Irish Cancer Society and Science Foundation Ireland since 2015.
BCNI is a collaborative network of clinicians, scientists, and population health experts with a shared interest in blood cancer research. Members of the national network are based at the NUI Galway/ University Hospital Galway, University College Cork/ Cork University Hospital, Trinity College Dublin/St James Hospital, Beaumont Hospital and the Mater Hospital, and the National Cancer Registry Ireland. The key investigators involved are Dr Eva Szegezdi, Dr Philip Murphy, Prof. Mary Cahill, Prof. Michael O’Dwyer, Prof. Paul Browne, Prof. Peter O’Gorman, Prof. Kerri Clough-Gorr and Dr John Quinn
The aim of BCNI is to provide blood cancer patients in Ireland with access to novel and innovative cancer treatments through the provision of early phase clinical trials. BCNI also collect information and samples from blood cancer patients in Ireland in order to improve our understanding and to uncover new ways to combat this disease..
HRB Stroke Clinical Trial Network Ireland
Stroke is the second leading cause of death in the world, the leading cause of new disability, and a major cause of dementia and health costs.
The HRB Stroke Clinical Trial Network Ireland is led by Professor Peter J Kelly, Mater University Hospital and University College Dublin. The Network will initially involve eight Irish hospitals, six leading universities, and all seven Hospital Groups, including colleagues from UCD, RCSI, Trinity College, UCC, NUI Galway, and University of Limerick. It will have strong links with international researchers in the UK, Europe, and North America. In addition to the HRB, other Network partners are the Irish Heart Foundation, who will fund new Stroke Research Nurses, and seven industry partners, who will fund education and training activities.
In the Network, Irish researchers in hospitals will:
- Join several new international trials of new treatments for emergency care, prevention, and recovery after stroke.
- Lead a new clinical trial aiming to prevent second strokes and heart attack after first stroke.
- Train new doctors, nurses, and therapists in how to perform safe high-quality clinical trials, and will work with patient groups and the private sector to bring new treatments to patients with stroke.
HRB Irish Critical Care Clinical Trials Network

About the ICC-CTN
The Irish Critical Care Clinical Trials Network (ICC-CTN), set up in 2015 by Professor Alistair Nichol, has become a leading critical care research network in Ireland and globally. By supporting ICUs throughout Ireland and Europe, the ICC-CTN has helped to facilitate the roll-out of global trials in ICUs across the island of Ireland, giving Irish patients access to novel treatments. With a large network of collaborators around the world, the ICC-CTN has been able to secure both national and international funding, which has helped to grow the ICC-CTN team and widen the scope of the trials and research activities they conduct and support. This work has made a significant contribution to ICU research, informing evidence-based clinical practices and guidelines on a global scale.
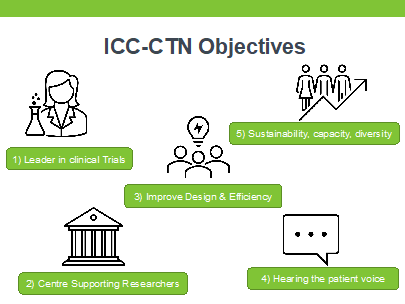
Strategic Objectives
After receiving a renewal of funding from the HRB CTN-2021 award scheme, the network has begun the expansion of projects and activities including leadership in national and international PPIE groups, Training, Education, Methodology & more. Their main objectives are:

Research Priority Areas
The core thematic research areas include 1) Pandemic Preparedness 2) Out of hospital Cardiac Arrest, 3) Trauma, 4) Acute Respiratory Failure, 5) Acute Renal Failure. The ICC-CTN conducts and supports clinical trials and observational, non-interventional and pilot/feasibility studies in these core areas and more, including sepsis, sedation and delirium, nutrition, and physiotherapy.

During the last seven years, the ICC-CTN have been involved in the coordination of over 19 ICU clinical studies that have delivered clinical practice changing results to more than 1600 Irish patients and more than 800,000 patients globally. The network works with 18 Irish, and more than 30 European hospitals as part of a global collaboration that spans 63 countries and multiple partnerships.

HRB Dementia Clinical Trials Network
ATLANTIC DiP (Diabetes In Pregnancy)
The ATLANTIC DiP research programme has focused on examining the outcomes of pregnancy for women with Type 1 and Type 2 Diabetes and the factors influencing these outcomes. The mission of ATLANTIC DIP is to improve the outcomes of pregnancy for women with Diabetes by promoting evidence based best practice before, during and after pregnancy.
Galway Diabetes Research Centre (GDRC)
GDRC is a joint venture between NUI Galway and Galway and Roscommon University Hospital Group, which bring together an interdisciplinary research consortium of active researchers and clinicians who have a track record in the field of diabetes. The centre is a hub for collaborative effort of researchers and clinicians, with a passion for this work, to provide a greater understanding of how diabetes develops and the underlying mechanisms, the development of new and better therapies for patients, and assessing health care interventions and delivery for patients.
HRB Diabetes Collaborative Clinical Trial-Network-Ireland (DCCTN)
The aim of the HRB Diabetes Collaborative Clinical Trial Network is to improve health and wellbeing for all patients with Diabetes through research and an active portfolio of ambitious multicentre trials on the island of Ireland. Regardless of geographical location or socioeconomic status, all patients will be facilitated to participate in relevant trials through a well-developed network of clinical sites supported by Clinical Research Facilities, Academic Institutions, and the National Clinical Care programme in Diabetes. This ambitious aim is supported by the individual network members who have considerable experience in investigator and industry led clinical trials. They can demonstrate individual success with national and international grant funding and the influence of their research on policy and guidelines. They have a broad international collaboration base with distinguished world experts in their fields. The network is building further on national investment in clinical research facilities (CRFs)/networks, bringing together academics, clinicians, health professionals, patients, industry, charities, and health care delivery networks. The network aims to collaborate fully with the existing CTNs. The network will have strong PPI input and will be overseen by the National HRB PPI hub, and trial designs will be robustly debated with the HRB TMRN and supported by core outcome sets (COSs) and Evidence Synthesis Ireland.
The network core working group will focus on development of (a) a research strategy (b) a governance structure (c) plan for interaction with industry (d) establishing a patient groups and public PPI group (d) sustainability plan with a focus on national and international grant applications, in addition to industry and SME fiscal and in-kind contributions. While they network members continue to put patients and patient improvement at the heart of diabetic clinical research.
Currently the network is in start-up phase, establishing sustainable governance structures, developing a consistent logo and brand identity, creating a website for network members, public and patient involvement and developing a research strategy. They can be contacted by emailing peter.rooney@nuigalway.ie.
Investigator Network for Inflammatory Bowel Disease Therapy in Ireland (INITIative)
INITIative is the first collaborative research network for Inflammatory Bowel Disease in Ireland. The network is open to clinical and scientific investigators with an interest in Crohn’s Disease and Ulcerative Colitis throughout the island of Ireland. Goals of the Network include:
- To foster collaboration and encourage multi-centre investigator initiated studies in Crohn’s Disease and Ulcerative Colitis in Ireland
- To attract and facilitate industry sponsored clinical trials in Crohn’s Disease and Ulcerative Colitis in Ireland in order to improve the access for patients in Ireland to investigational drugs and therapies
- To facilitate participation by Irish IBD investigators in European research consortia and networks
- To attract funding for and investment in research infrastructure to support research in Crohn’s Disease and Ulcerative Colitis in Ireland
- To foster all Ireland, European and Global collaboration in IBD research and improve the profile of IBD related research taking place in Ireland
Current INITIAtive projects include several investigator initiated studies: GOAL-ARC and HARP-X. GOAL-ARC is a nationwide multi-centred investigator initiated randomized control trial to evaluate the use of personalized golimumab (GLM) dose adjustment in UC. The primary objective is to ascertain if dose adjustment of GLM, based on GLM drug levels and FCP levels, results in higher response and remission rates than standard dosing.
HARP-X is a pilot study that will determine the effect of tofacitinib maintenance therapy on endoscopic response in subjects with active, chronic, antibiotic dependent or refractory pouchitis.
INITIative continues to participate in numerous observational studies, including I-CARE, the first European prospective cohort study that will provide unique information on the long-term use of recommended therapy in IBD.
A key priority of the network is to expand research into non-pharmacologic interventions, including psychological and dietary interventions, nursing interventions, and methods to address patient quality of life. There are currently several studies in the pipeline looking at these important aspects of IBD. In addition, the network’s research priorities are targeted for understudied patient groups, including patients with stomas, proctitis and pouchitis that are generally excluded from industry sponsored studies.
For further information please contact:
Glen Doherty MB BCh PhD FRCPI FEBGH
Senior Clinical Lecturer, University College Dublin and Consultant Gastroenterologist and Physician, Centre for Colorectal Disease, St. Vincent’s University Hospital
Annie Coe, BSN, MScResearch Nurse & INITIative Coordinator
Centre for Colorectal Disease, St. Vincent’s University Hospital
Email: c.coe@svuh.ie
The Irish Network for VTE Research
The Irish Network for VTE Research (INViTE) is an Irish, patient-oriented research network aimed at developing and participating in excellent National and International venous thromboembolism (VTE)-related research. We are honoured to work with the patient group Thrombosis Ireland (http://thrombosisireland.ie) at every step along the way of study concept, design and development
INViTE was launched on 12th September 2018 by the Irish Minister for Business, Enterprise and Innovation Heather Humphries in Croke Park, Dublin and is a member of the prestigious INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com).
Partners
Patient organization Thrombosis Ireland (http://thrombosisireland.ie/)
Clinician organizations:
VTE Ireland; Irish Association of Emergency Medicine
Translational research groups/Centres:
UCD Conway SPHERE Research Group (http://www.ucd.ie/conway/research/researchgroups/ucdconwaysphere/); Irish Centre for Vascular Biology (http://www.rcsi.ie/ICVB)
Hospital partners:
- University Hospital Limerick
- Rotunda Hospital Dublin
- National Maternity Hospital, Holles Street, Dublin
- St Vincent’s University Hospital Dublin
- Our Lady of Lourdes Hospital Drogheda
- St James’s Hospital Dublin;
- Beaumont Hospital Dublin
- Cork University Hospital
- Mater Misericordiae University Hospital Dublin
- University Hospital Galway
- Our Lady’s Hospital Navan
- Sligo University Hospital
- Midland Regional Hospital Tullamore
- Coombe Women and Infants Hospital, Dublin
- Belfast City Hospital (Due to join in 2021)
University partners:
University College Dublin, University of Limerick, Royal College of Surgeons in Ireland, University College Cork, Trinity College Dublin, (2021: Queens University, Belfast)
CRCs: University of Limerick CRU, RCSI, UCD, TCD CRCs
Inclusion Health Service, St James’s Hospital, Dublin
Department of Health Intelligence R&D, Irish Health Services Executive
Hospital Groups: Ireland East Hospital Group
INVENT-VTE (International Network of Venous Thromboembolism Clinical Networks; www.invent-vte.com)

ID-CTNI: Infectious Diseases Clinical Trials Network Ireland
I
ID-CTNI is led by Professor Paddy Mallon, St Vincent’s University Hospital and University College Dublin (UCD). The ID-CTNI network is hosted at the UCD Clinical Research Centre and supported by the UCD Centre for Experimental Pathogen Host Research (CEPHR).
The ID-CTNI network brings together a team with expertise in leading, directing and assisting Infectious Diseases clinical trials and investigations, both investigator and industry led. The multidisciplinary network comprises academic clinicians and researchers from:
- University College Dublin/St Vincent’s University Hospital & Mater Misericordiae University Hospital
- University College Cork/Cork University Hospital
- Trinity College Dublin/St James’s Hospital
- Royal College of Surgeons in Ireland/Beaumont Hospital
- University of Galway/Galway University Hospital
The objectives of the ID-CTNI include:
Implement a governance structure that improves Infectious Diseases clinical trial design, methods, and coordination in Ireland
Integration of existing investigator-initiated clinical trials and the implementation of new multicentre clinical trials.
Patient involvement will play a central role in the network and planning of clinical trials.
The expertise within the ID-CTNI enables better integration of resources and training of new researchers in Infectious Diseases, this will better place ID-CTNI research internationally.
Along with other clinical trial networks, Align and integrate with national strategic initiatives in Infectious Diseases to be better prepared for future pandemics
The unprecedented focus on Infectious Diseases arising from the COVID19 pandemic is providing opportunities for the ID-CTNI to develop international clinical trials with other clinical trials networks.
Current Multi-site Clinical Trials include:
EU-COVAT-2 BOOSTAVAC (COVID-19 Booster Vaccine Trial)
An International Multicentre, Phase 2, Randomised, Adaptive Protocol to determine need for, optimal timing of and immunogenicity of administering a 4th homologous mRNA vaccination dose against SARS-CoV-2 in the general population (18+ years) already vaccinated against SARS-CoV-2
COVIRL-002 – Tocilizumab for management of severe, non-critical COVID-19 infection.
An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive decline in respiratory function and evolving systemic inflammation on time to intubation, non-invasive ventilation and/or all-cause mortality.
SWIFT Study – Semaglutide’s Efficacy in Achieving Weight Loss for Those With HIV.
A randomised, controlled, parallel group, open-label definitive intervention trial evaluating the impact of a 28-week course of weekly, subcutaneous Semaglutide alongside dietary and exercise advice on weight loss, inflammation and immune function in obese people living with HIV on stable antiretroviral therapy compared to dietary and exercise advice alone.
A new Monkeypox Vaccine Trial study to be started soon.
For queries please contact:
Dr Polina Smovolyk, Project Manager.
Email: <polina.smolovyk@ucd.ie>
Motor Neurone Disease
Research Motor Neurone (RMN) was founded in 2007, for the purpose of promoting and facilitating research into the causes and treatments of motor neurone disease (MND), also known as ALS. RMN also strives to increase awareness of this incurable disease at both a national and international level. Ongoing research is needed to discover the cause, treatment and methods of improving quality of life for MND sufferers and their families.
Neurology Research Group, St. Vincent’s University Hospital
The Neurology Research Group in St. Vincent’s University Hospital (SVUH) was established over 15 years ago. It has expertise and experience running numerous clinical pharmaceutical trials, academic research studies – both clinical and basic science, and interventional studies with allied health professionals. Several national neurology registries and research databases are also co-ordinated from the SVUH site.
Their particular areas of excellence are neuroinflammatory disorders, in particular multiple sclerosis, movement disorders and cognitive neurology. They have a dedicated clinical facility and can run clinical trials in all other areas of neurology. Their principal Investigators are recognised as international experts in their field of research and are experienced national principle investigators for many clinical trials. In addition to four consultant neurologists, they have a dedicated research team consisting of neurology research registrars, clinical research nurse manager/ coordinator, 3 clinical research nurses and a research assistant. As well as dedicated clinical facilities they have office space for external monitors, laboratory facilities on site for sample processing and access to neuroimaging, neurophysiology, neuropsychology, neuropsychiatry and neuro-ophthalmology services to name but a few. They have on-going collaborations with a number of basic neuroscience and bioengineering groups in Ireland and internationally.
For further information please contact:
Sinead Jordan, Neurology Research Nurse Manager
Email: s.jordan@st-vincents.ie
Phone: (01) 2213592

Irish Network for Children’s Clinical Trials
In4kids is a HRB funded national paediatric clinical research network hosted by the INFANT Centre in University College Cork (UCC). The network’s vision is for better medicines, medical devices & interventions for babies, children & young people in Ireland. Children are not just small adults – they have distinct physiologic, developmental, and psychological characteristics. They deserve and must have the same access to high quality, evidence-based (from paediatric studies) health care as adults..
Through increased and enhanced collaboration amongst the paediatric research community in Ireland, In4kids will develop the national capacity for high-quality, ethical paediatric clinical research.
They welcome all of the Paediatric research community across Ireland to join them, by becoming members of the network. Benefits of this are access to the members only platform of bespoke supports and tools for conducting paediatric research, increased opportunities to collaborate on national clinical research projects and being kept informed on the recent advances in national and international paediatric research.
More information on In4kids membership is available here.
Follow them on Twitter and LinkedIn.
Subscribe to their newsletter.
Visit their website for further information.
For general enquiries email: info@in4kids.ie
A research centre focused entirely on pregnancy, birth and early childhood.
INFANT is hosted by University College Cork (UCC), Ireland, and co-located in Cork University Hospital and Cork University Maternity Hospital. INFANT was established in 2013, building on over a decade of award-winning translational research with a mission to deliver pioneering translational research to improve health outcomes for mothers and babies.
INFANT’s research is underpinned by leadership in cross-disciplinary clinical and scientific research disciplines. From pregnancy to early childhood, INFANT conducts research in three core areas:
(1) Neuroscience and Development
(2) Foetal and Maternal Health and
(3) Nutrition and Growth.
INFANT has made many world-first scientific breakthroughs including: the development of an algorithm that detects seizures in new-born babies; the discovery and validation of biomarkers to detect brain injury in new-born babies, and the development of early predictive biomarkers for preeclampsia. Read more here.
Research at INFANT is led by a multidisciplinary team of investigators comprised of clinicians, scientists, and engineers with the support of the INFANT Operations and Clinical Research Teams.
Follow them on Twitter, Instagram, Facebook and LinkedIn.
Visit their website for more information.
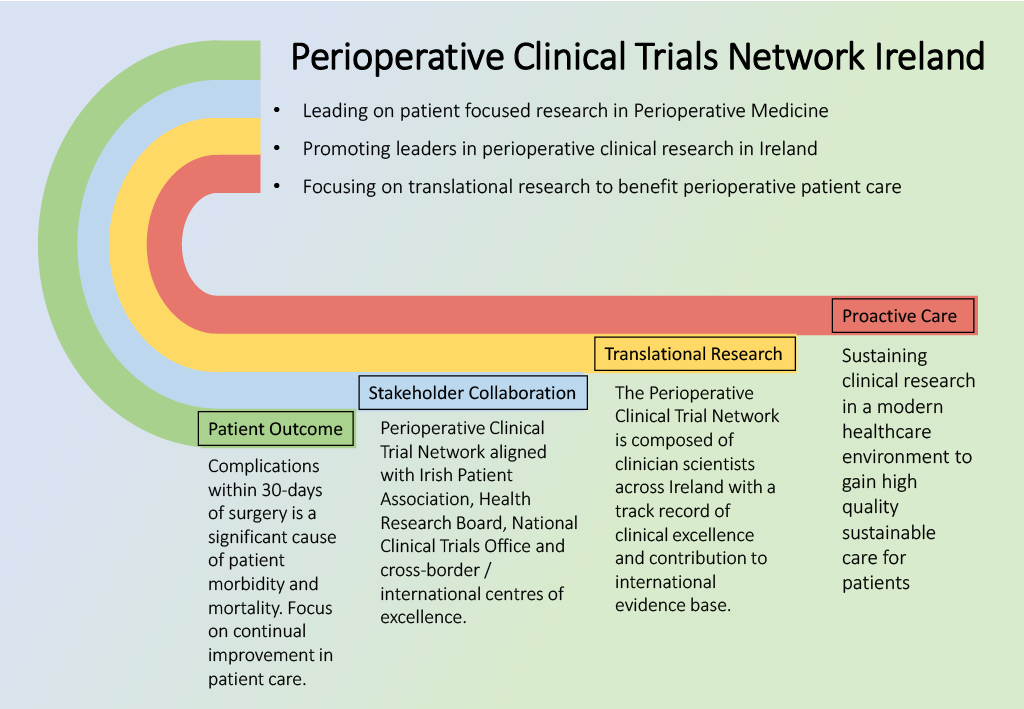
Perioperative Clinical Trials Network Ireland
For all queries please contact:
Prof. Donal J. Buggy, Network Manager & Professor of Anaesthesiology & Perioperative Medicine, UCD.
Email: donal.buggy@ucd.ie

HRB Primary Care Clinical Trial Network Ireland
The HRB Primary Care Clinical Trials Network Ireland (CTNI) aims to support the creation of high-quality clinical evidence which improves patient outcomes in Irish primary care. To achieve this, they provide supports to researchers to develop and conduct clinical trials in primary care that address important and common problems. They then support the successful translation and implementation of this evidence into healthcare policy and practice.
The HRB Primary Care CTNI was established in 2015 as a collaborative partnership between NUI Galway, the Royal College of Surgeons in Ireland, Queen’s University Belfast, the Association of University Departments of General Practice in Ireland, and the Irish College of General Practitioners. Since establishment, it has supported more than 30 clinical studies, recruited almost 4,000 patients, leveraged funds of over €19,000,000, and published in leading international journal such as the New England Journal of Medicine, the Lancet, and the British Medical Journal.
In 2021, the HRB Primary Care CTNI was successful in securing funding for the next 5 years of operations, with a focus on achieving the following strategic objectives:
- To maximise the successful delivery of primary care trials in Ireland.
- To continue to build capacity for world-class clinical trials in Irish primary care, through the provision of financial supports to early career researchers, the promotion of primary care research education and dissemination, and the provision of seed funding to develop a roadmap for implementation of a national research IT infrastructure.
- To develop an agenda for Irish primary care clinical trials research, by leading a priority setting partnership to develop the ‘Top 10’ research priorities in chronic disease management, and a core outcome set (COS) for future trials in this area.
- To enhance patient and public involvement (PPI) in primary care research in Ireland, by continuing to grow the capacity of the Primary Care CTNI PPI group, including PPI in network oversight and portfolio development, and continuing our synergistic relationship with the national PPI network.
For more information, contact the Primary Care CTNI directly by emailing primarycaretrials@universityofgalway.ie
Irish College of General Practitioners (ICGP)
The research unit of the ICGP aims to develop and support research and audits in general practice. The overall aim of the research unit is to contribute to the knowledge base of general practice and to support evidence-based practice. The ICGP Research Ethics Committee, granted formal recognition on 17th May 2005, reviews both CTIMP and non-CTIMP projects. The College provides research training and guidance to GPs and GP trainees through in a variety of formats and is a partner on the Primary Care Clinical Trials Network.
For all queries please email: research@icgp.ie
HRB Rare Disease Clinical Trial Network:
The RD-CTN, a HRB research network, led by Prof Rachel Crowley and Prof Cormac McCarthy at University College Dublin, aims to enhance rare disease care and outcomes, with the following objectives:
- Act as a collaborative hub for trials in rare diseases
- Facilitate and support the conduct of trials in rare diseases
- Increase the opportunities for rare disease patients to access high-quality clinical trials.
This will result in increased in patient engagement; more trial opportunities for rare disease patients; increased opportunity for investigator-initiated trials; significant industry investment into Irish healthcare; enhanced knowledge of rare disease through patient focused research and parallel translation scientific studies.
Link to HRB announcement: https://www.hrb.ie/news/news-story/article/hrb-announces-e1-million-investment-for-rare-disease-clinical-trial-network-on-rare-disease-day-2022-1/
Website: rarediseaseresearch.ie
Current Clinical Trials:
TOPAZ:
TOPAZ is a randomised open-label clinical trial for people with osteogenesis imperfecta (OI). Osteogenesis Imperfecta (OI) is a rare genetic condition present from birth and is frequently called ‘brittle bone disease’. The study aims to investigate whether a treatment with a drug called teriparatide (TPTD) followed by treatment with another drug called Zoledronic acid (ZA) reduces the risk of broken bones occurring in people with OI.
Study design:
Participants will be randomised in a 1:1 ratio to receive treatment with teriparatide (TPTD) for 2 years followed by an infusion of ZA or to receive standard care for the duration of the study. All participants will be followed up for a total duration of between 2 and 5 years.
Inclusion criteria:
- Adult patient aged 18 years and over with a clinical diagnosis of osteogenesis imperfecta.
- Patients willing and able to consent and comply with the study protocol
Type of Study: Phase III/IV
Study Status: Ongoing
Principal Investigator: Prof Rachel Crowley
Contact: Suzanne.mccormack@ucd.ie
IMPALLA-2:
IMPALLA – 2 is a randomized, double-blind, placebo-controlled multicentre clinical trial investigating efficacy and safety of inhaled molgramostim (rhGM-CSF) in patients with Autoimmune Pulmonary Alveolar Proteinosis aPAP. Autoimmune PAP is caused by an immune system malfunction due to IgG antibodies that block the granulocyte-macrophage colony stimulating factor (GM-CSF) effect. GM-CSF is a protein that regulates clearance of surfactant (a mix of protein and fat) by alveolar macrophages. The surfactant accumulates in the air sacs of the lungs and eventually lead to an inability to breath. The aim of this trial is to confirm if Molgramostim Nebulizer Solution (inhaled molgramostim) improves function of the lungs in patients with autoimmune pulmonary alveolar proteinosis (aPAP).
Study design:
The trial will include 2 periods; a double-blind treatment period consisting of up to 8 trial visits and an open-label follow-up period consisting of up to 5 trial visits.
Type of Study: Phase III/IV
Study Status: Ongoing
Principal Investigator: Prof Cormac McCarthy
Contact: Suzanne.mccormack@ucd.ie
__________________________________________________________________________________________
For any other query, contact:
Suzanne McCormack
Rare Disease Clinical Trial Network Manager
Email: Suzanne.mccormack@ucd.ie
Tel: 086 8573927
The Rare Kidney Disease (RKD) Registry and Biobank
The Rare Kidney Disease (RKD) Registry and Biobank was established in 2012 to facilitate research into the rare auto-immune condition ANCA-associated vasculitis (AAV) in Ireland. Development of a networked collaboration across all the major hospital sites in the country enabled effective recruitment of patients presenting with the disease, and the capture of their clinical data and linked blood and urine samples. After joining the pan-European initiative ERN-RITA in 2022, the resource was renamed the RITA-Ireland Vasculitis (RIV) Registry and Biobank, contributing data to the RITA registry. Hosted within the School of Medicine at TCD, the Registry and Biobank today comprises over one thousand vasculitis recruits, many of whom have been tracked longitudinally, and represents one of the largest bioresources of it’s kind globally. The Principal Investigator is Professor Mark Little.
Find out more at https://www.tcd.ie/medicine/thkc/our-research/rita-ireland-vasculitis-riv-registry-and-biobank/ & https://ern-rita.org/rita-registry/general-info/
Respiratory and Asthma Research Network
The INCA™ Studies are a suite of clinical investigations. The focus of our research is in developing a novel technology which can be used in clinical practice as an objective assessment of patient adherence to inhaled therapy.
For further information please contact:
Elaine Mac Hale, Programme manager: Elainemachale@rcsi.ie
Arthritis Research Coalition
A recent report assessing rheumatology research excellence, measured by the number of citations per article published between 1996 and 2010, ranked Ireland 1st of the 35 richest countries. Major research outputs have included molecular studies on the pathogenesis of common rheumatic diseases, the discovery and validation of prognostic biomarkers, and clinical studies on novel therapies. These have relied on the recruitment of highly characterized patients populations and by the development of linked biorepositories of blood and synovial tissue.
Despite these successes the research activity has been reliant on a fragmented and poorly funded infrastructure. The development of national rheumatology research networks in many European countries including Britain, Sweden and The Netherlands highlights their value. The establishment of Arthritis Ireland chairs, and the development of a Dublin Centre of Excellence, will form the foundation of national rheumatology research strategy, however, the development of a research nurse network will seek to engage all clinicians, co-ordinate all research activities and ensure that Ireland remains on top.
The primary aim is recruit patients with common rheumatic diseases. and obtain biosamples that will underpin clinical research. A secondary aim is to increase national involvement in clinical trials of novel therapeutic agents.
The consortium lead is Prof Gerry Wilson. The leads by area are as follows:
Rheumatoid Arthritis – Prof Doug Veale
OA/Crystal arthritis – Prof Geraldine McCarthy
Spondyloarthropathies – Dr Barry O’Shea
Connective Tissue Diseases – Dr Grainne Murphy
Paediatrics – Dr Orla Killeen
Metabolic Bone- Dr John Carey
For further information:
Email: rheumatology@ucd.ie
National Surgical Research Support Centre (NSRSC)
The RCSI-NSRSC was set up to establish an all-island Irish Surgical Trial Network to enhance the collaboration and coordination of surgical research and trial activity in Ireland.
Objectives of the Network
Encourage and facilitate surgical trials and research to embed a culture of research in clinical practice.
Establish a trial portfolio to generate high quality evidence to inform policy and set professional standards & guidelines for Irish surgery to improve patient outcomes.
Provide training and education to surgical trainees to produce the next generation of academic leaders in surgery.
Showcase surgical trial activity to establish Ireland as an attractive place to conduct innovative surgical trials.
To provide every patient access to high quality surgical trials and innovative cutting-edge technology across the island of Ireland.
Supports offered
The NSRSC acts as a point of contact, local sponsor and trial coordinating centre for Investigators and international sponsors who would like to open trials here in Ireland. We offer access to a wide network of surgeons, surgical trainees, allied healthcare professionals and scientists participating in surgical research.
Types of surgical research supported
The centre supports trials across all surgical specialities and thematic areas.
Currently the NSRSC are supporting multi-centre Investigator-led Randomised Controlled trials across a range of areas including diabetic foot disease, appendicitis, bariatric surgery, wound healing devices, varicose vein surgery, cancer and chronic subdural haematoma.
One key area of focus is on infection prevention in patients undergoing a variety of surgical procedures.
The network also supports and coordinates international collaborative surgical trials, pilot/feasibility studies, observational and non-interventional research studies.
Check out the website, Twitter and LinkedIn or contact the NSRSC:
Web: www.rcsi.com/NSRSC
Twitter: @RCSI_NSRSC
LinkedIn: www.linkedin.com/company/nsrsc
Email: NSRSC@rcsi.ie