ECRIN – Connecting to Europe
ECRIN – Connecting to Europe
The European Clinical Research Infrastructure Network (ECRIN) is a public, non-profit organisation that links scientific partners and networks across Europe to facilitate multinational clinical research. As of 2013, ECRIN has the legal status of a European Research Infrastructure Consortium (ERIC). ECRIN links the resources and capacities of national networks across Europe to increase access to patients and medical expertise.
ECRIN’s core office is based in Paris, and ECRIN works with European Correspondents (EuCos) across Europe, national networks of clinical trial units (CTUs)/clinical research facilities (CRFs), as well as numerous European and international stakeholders involved in clinical research.
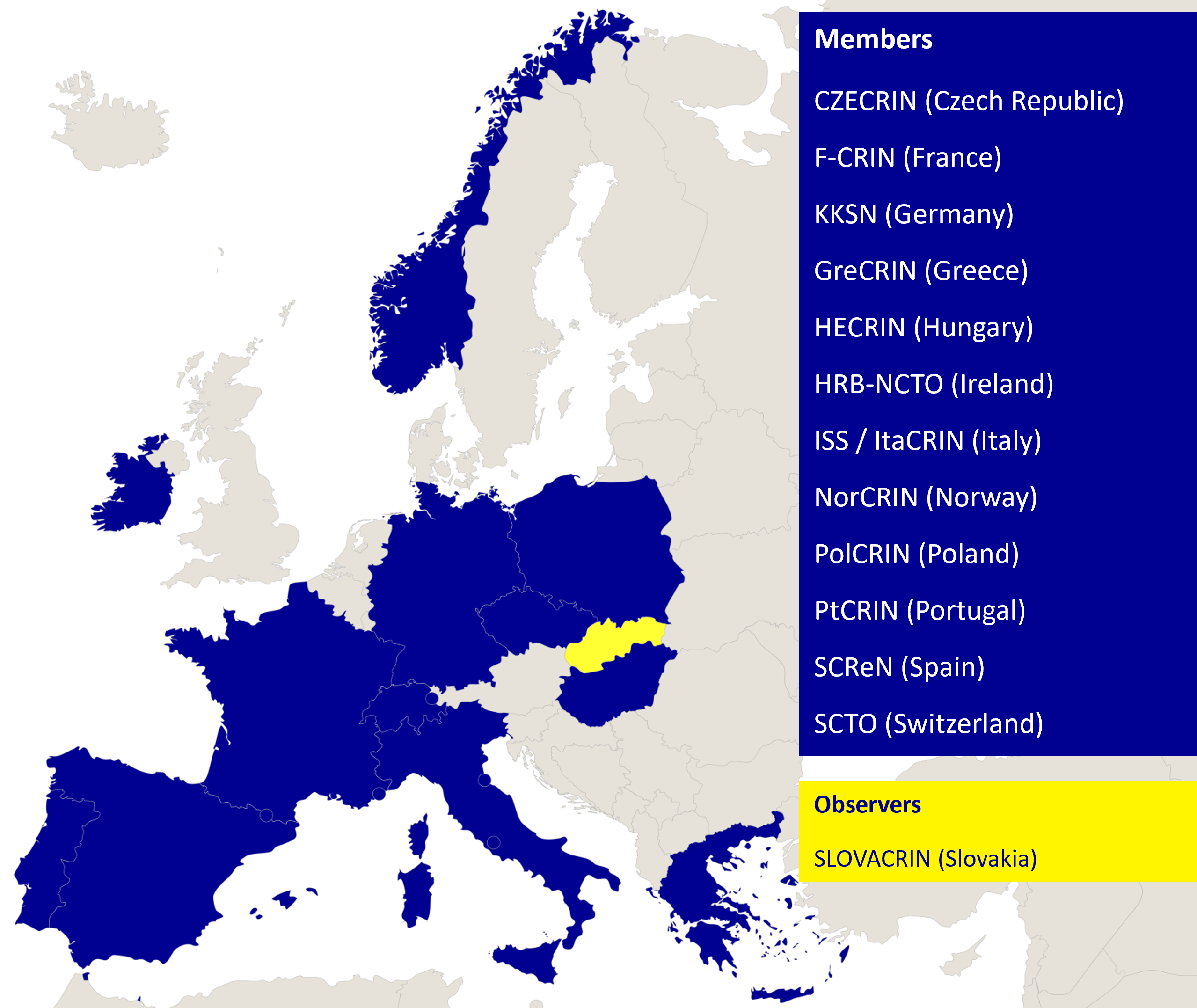
ECRIN’s organisational model is based on country membership. Countries can either be full Members or Observers. Current Members include Czech Republic, France, Germany, Greece (joined May 2023), Hungary, Ireland, Italy, Norway, Poland, Portugal, Spain and Switzerland (acquired ECRIN member status from being an observer as of June 2023), and Observers include Slovakia.
Ireland became a full member of ECRIN on November 20th, 2018. As a member of ECRIN, Irish researchers can benefit from a full range of ECRIN services for multinational clinical research study preparation, protocol evaluation and study management.
The European Clinical Research Infrastructure Network (ECRIN) is a public, non-profit organisation that links scientific partners and networks across Europe to facilitate multinational clinical research. As of 2013, ECRIN has the legal status of a European Research Infrastructure Consortium (ERIC). ECRIN links the resources and capacities of national networks across Europe to increase access to patients and medical expertise.
ECRIN’s core office is based in Paris, and ECRIN works with European Correspondents (EuCos) across Europe, national networks of clinical trial units (CTUs)/clinical research facilities (CRFs), as well as numerous European and international stakeholders involved in clinical research.
ECRIN’s organisational model is based on country membership. Countries can either be full Members or Observers. Current Members include Czech Republic, France, Germany, Hungary, Ireland, Italy, Norway, Poland, Portugal and Spain, and Observers include Slovakia and Switzerland.
Ireland became a full member of ECRIN on November 20th, 2018. As a member of ECRIN, Irish researchers can benefit from a full range of ECRIN services for multinational clinical research study preparation, protocol evaluation and study management.
ABOUT Irelands ECRIN Membership
ABOUT Irelands ECRIN Membership
Ireland became a full member of ECRIN on November 20th, 2018.

As a member of ECRIN, Irish researchers can benefit from a full range of ECRIN services for multinational clinical research study preparation, protocol evaluation and study management
To access ECRIN services contact the Irish EuCos ncto@ucc.ie
To learn more about ECRIN, please see the video below
PROPOSAL DEVELOPMENT STAGE
ECRIN can offer more extensive support during the proposal development stage when it is involved as a project partner. Support may be required for EU funding proposals such as Horizon Europe and Innovative Health Initiative (IHI). Researchers should contact the Irish EuCos early in the proposal development process (ideally 3 to 6 months prior to the submission deadline).
PROPOSAL DEVELOPMENT STAGE
ECRIN can offer more extensive support during the proposal development stage when it is involved as a project partner. Support may be required for EU funding proposals such as Horizon Europe and Innovative Health Initiative (IHI). Researchers should contact the Irish EuCos early in the proposal development process (ideally 3 to 6 months prior to the submission deadline).
Examples of the types of support available at the proposal stage include:
Design and methodology support
Advice on EU funding sources
Budget development and cost estimation
Regulatory, ethics and insurance requirements
Medical expertise and support
Strategies for site selection and patient recruitment
Design and methodology support
Advice on EU funding sources
Budget development and cost estimation
Regulatory, ethics and insurance requirements
Medical expertise and support
Strategies for site selection and patient recruitment
POST FUNDING AWARD – STUDY PREPARATION
Once the study has been awarded funding ECRIN can provide the following support during the study preparation phase:
POST FUNDING AWARD – STUDY PREPARATION
Once the study has been awarded funding ECRIN can provide the following support during the study preparation phase:
Protocol peer review
Feasibility and risk assessment
Protocol peer review
Feasibility and risk assessment
STUDY IMPLEMENTATION
ECRIN offers support during the study implementation phase in various areas such as:
STUDY IMPLEMENTATION
ECRIN offers support during the study implementation phase in various areas such as:
Study management and coordination
Regulatory and ethical submissions
Selection and provision of qualified resources
Pharmacovigilance (adverse event reporting)
Data management
Monitoring
Study management and coordination
Regulatory and ethical submissions
Selection and provision of qualified resources
Pharmacovigilance (adverse event reporting)
Data management
Monitoring



